RESEARCH AREAS
– Dimple Array on Surfaces of Channels
– Investigations of Confined, Millimeter-Scale, Unsteady Laminar Impinging Slot Jets
– Aerodynamic Losses and Mixing Losses from Turbine Airfoils
– Impingement Cooling
– Internal Cooling – Surface Heat Transfer Augmentation
– Miniature and Micro-Scale Pumps
– Surface Roughness
– Electronics Cooling
– Transitional Flows in Curved Channels
– Film Cooling
– Flow and Heat Transfer on and Near a Transonic Turbine Blade Tip
– Slip Phenomina in Micro-Fluidic Devices
– Buoyancy-Driven Continuous SPLITT Fractionation: A New Technique for Separation of Microspheres
– Investigations of Full-Coverage Film Cooling
– Shock Wave Boundary Layer Interactions
– Double Wall Cooling
– Elastic Turbulence
– Surface Roughness Effects on Impingement Jet Array Surface Heat Transfer
– Dean Flow Dynamics in Low-Aspect Ratio Spiral Microchannels
Buoyancy-Driven Continuous SPLITT Fractionation: A New Technique for Separation of Microspheres
Buoyancy-Driven Continuous SPLITT Fractionation: A New Technique for Separation of Microspheres (J. Storey, P. Douglas, P. M. Ligrani, and K. M. Morten), Separation Science and Technology, Vol. 44, No. 9, pp. 1895-1922, January 2009. DOWNLOAD COPY OF PAPER
FIGURES
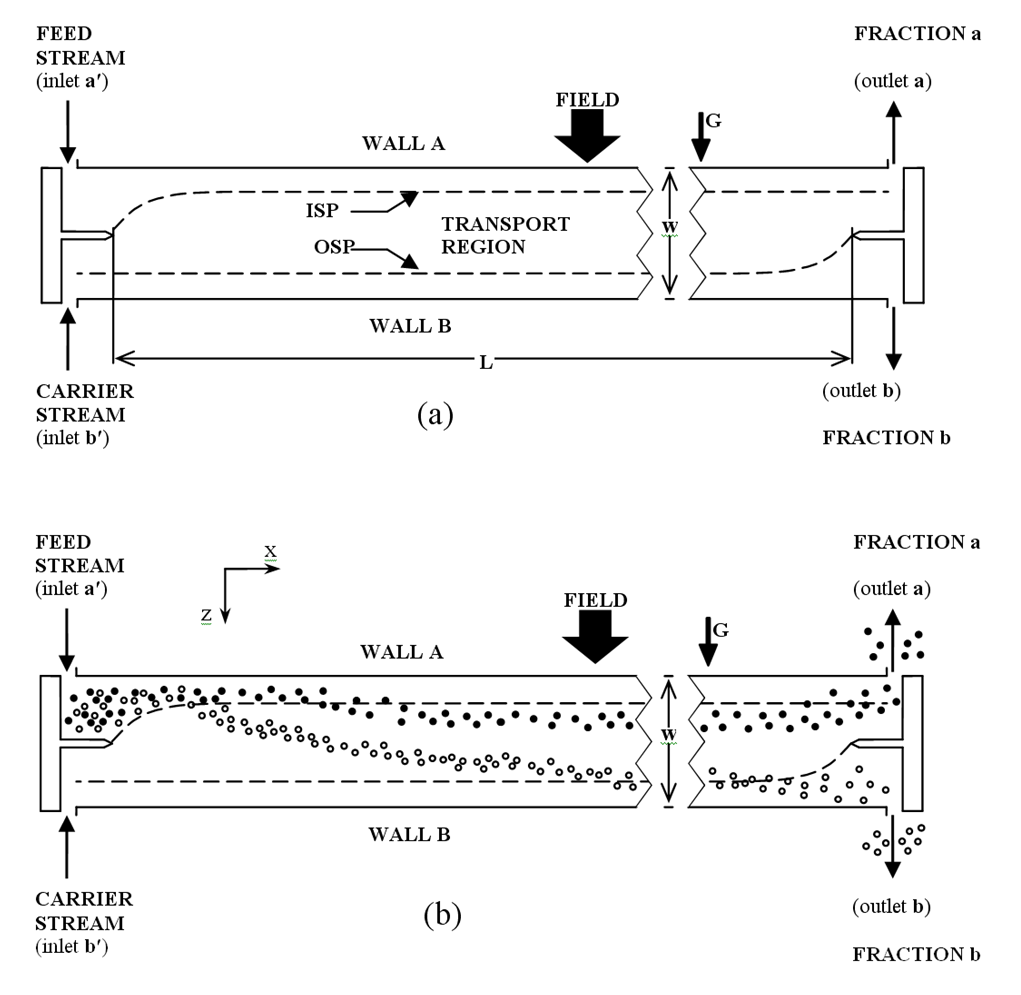 Schematic view of a SPLITT cell operating in sedimentation transport mode, showing (a) the overall layout of the cell, and (b) example particle trajectories during operation.
Schematic view of a SPLITT cell operating in sedimentation transport mode, showing (a) the overall layout of the cell, and (b) example particle trajectories during operation.
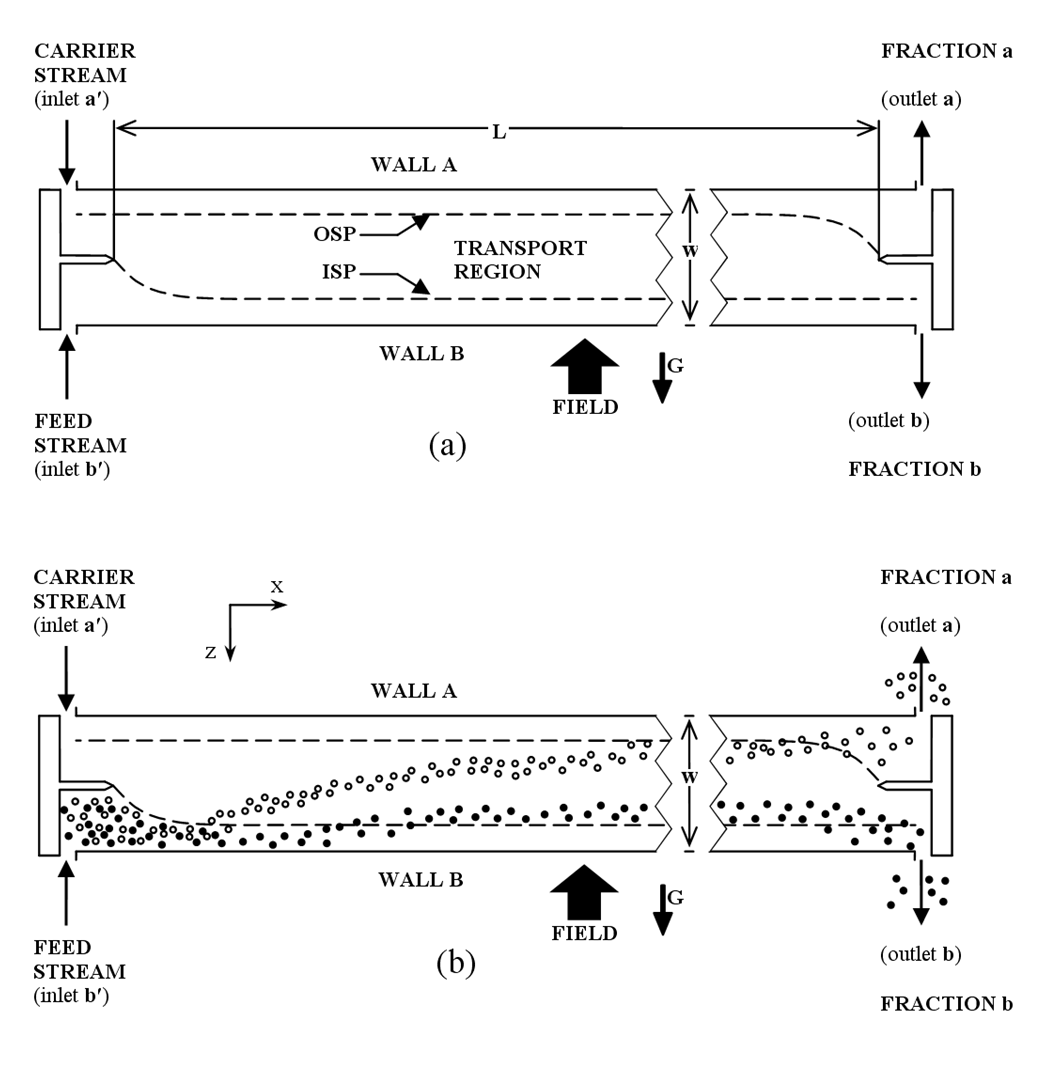 Schematic view of a SPLITT cell operating in buoyancy-driven transport mode, showing (a) the overall layout of the cell and (b) example particle trajectories during operation.
Schematic view of a SPLITT cell operating in buoyancy-driven transport mode, showing (a) the overall layout of the cell and (b) example particle trajectories during operation.
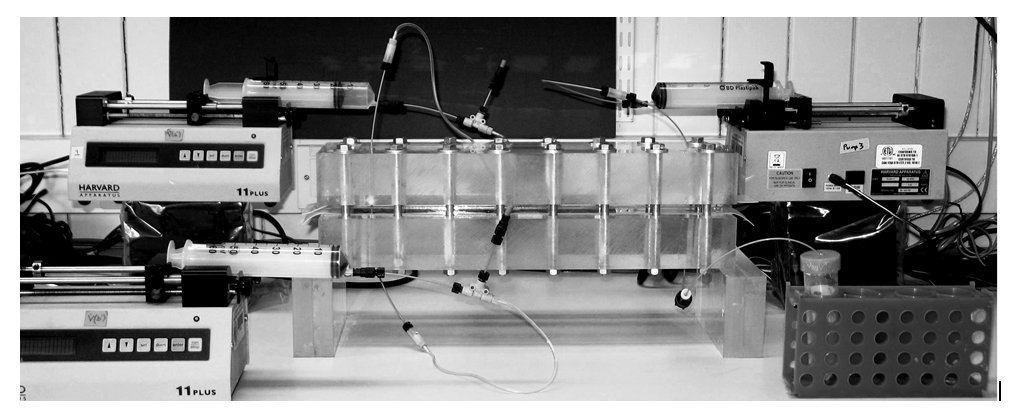 Photograph of the cell for the sedimentation-driven and buoyancy-driven experiments.
Photograph of the cell for the sedimentation-driven and buoyancy-driven experiments.
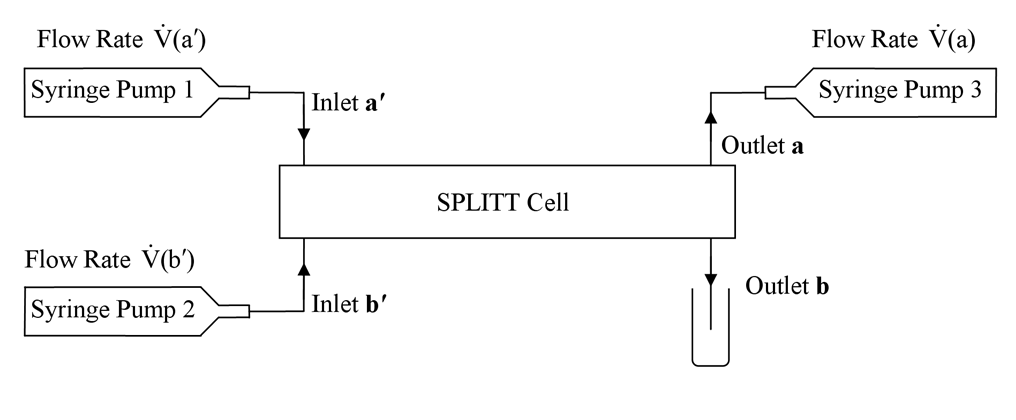
Schematic diagram showing flow connections to and from the cell, and the arrangement of the syringe pumps.
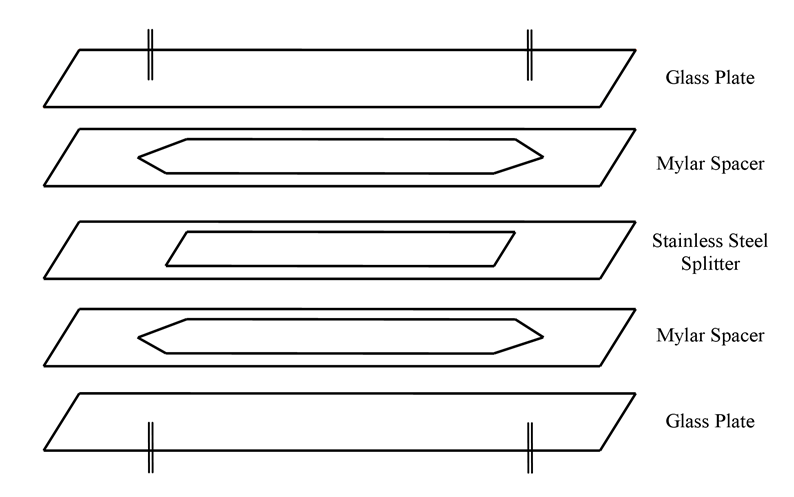 Schematic diagram showing an overview of the cell construction and layout.
Schematic diagram showing an overview of the cell construction and layout.
